Introduction
The last decade has witnessed significant advances in the development of biomarkers in oncology that play a critical role in the understanding of molecular and cellular mechanisms that drive tumor initiation, maintenance, and progression. Clinical molecular diagnostics and biomarker discoveries in oncology are advancing rapidly as investigators begin to understand the complex mechanisms that transform a normal cell into an abnormal one. These discoveries have fueled the development of novel drug targets and new treatment strategies. The standard of care for patients with advanced-stage cancers has shifted away from an empirical treatment strategy based on the clinical-pathological profile to one in which a biomarker-driven treatment algorithm based on the molecular profile of the tumor is used.1 Recent advances in multiplex genotyping technologies and high-throughput genomic profiling by next-generation sequencing (NGS) make possible the rapid and comprehensive analysis of the cancer genome of individual patients, even when derived from very little tumor biopsy material.
Predictive biomarkers can be helpful in matching targeted therapies with patients and in preventing toxicity associated with standard systemic therapies. Prognostic biomarkers identify somatic germline mutations, changes in DNA methylation, and elevated levels of microRNA and circulating tumor cells in the blood.1 Predictive biomarkers using molecular diagnostics to evaluate benefits that can be achieved through targeted therapy are successfully being used in clinical practice of personalized therapy for the treatment of several cancers. However, a number of challenges are associated with incorporating biomarker testing in the management of patients with cancer, and guidance in “what works best” is of interest to many oncology practices, both at academic centers and in community practice settings.
To this end, best practices for incorporating molecular biomarker testing in the delivery of precision therapy in lung cancer were the focus of a conversation between Roy S. Herbst, MD, PhD, Chief of Medical Oncology and Director of the Thoracic Oncology Research Program at the Yale Comprehensive Cancer Center, New Haven, CT, and David L. Rimm, MD, PhD, Professor, Department of Pathology, Director of Pathology Tissue Services, and Director of Translational Science in Pathology at the Yale University School of Medicine, New Haven, CT. These 2 experts shared their perspectives on how they work together as part of the cancer care team to deliver optimal personalized care to their patients. Many of their insights can be applied broadly to both academic- and community-based practices striving to meet the challenge of effectively incorporating biomarker testing into their treatment of patients with lung cancer.
Impact of Biomarker Testing on Cancer Therapy
Dr Herbst discussed his approach as an oncologist. “I do not see a patient until he or she has had biomarkers done. In lung cancer, it is critical that we know a patient’s status for EGFR, ALK, ROS1, and RET—actually, more than ever for RET—given some of the new inhibitors that are coming down the pike, and also PD-L1 [programmed death-ligand 1]. We need to know whether a patient has a particular molecular driver and then treat him or her appropriately. I find the PD-L1 status to be important. Even now, with so many chemotherapy combinations being used, it is still important to know PD-L1 status.”
Dr Rimm offered the pathologist perspective. “I think this is an exciting time in pathology. Historically, we have given a pathologic or histologic diagnosis. Now, we need to know more than that to manage patients, more so in lung cancer than in other cancers. It is not just the DNA sequencing information. We need to provide oncologists and their colleagues with the EGFR status of patients before they even see them, but also other genes that can be targeted by specific drugs. In fact, at every meeting, it seems as though there is a new gene that we need to provide. We have to be nimble in the way we provide the genes and be ready to provide more genes. What was a real breakthrough just a few years ago was with immune therapy; there is actually a companion diagnostic that is an immunohistochemistry companion diagnostic. This is exciting for us. That is why we brought online the FDA-approved companion diagnostic for a PD-L1 assessment for patients with lung cancer. We were one of the first centers to use the test routinely, certainly in our area, but even nationally. I think most sites are now doing it—probably 80% to 90% of the sites are at least providing it. They need to have this information for the oncologists, and the pathologist is the one that provides the information.”
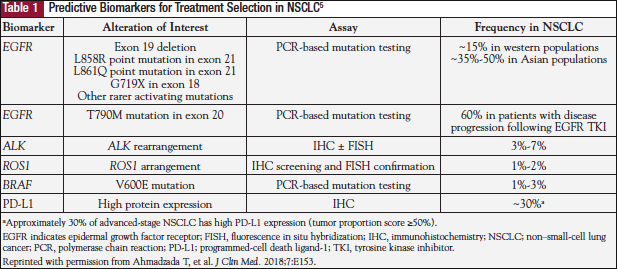
This initial exchange between the medical oncologist and the pathologist set the stage for a discussion on how the use of molecular biomarkers affects clinical practice, particularly as it applies to immunotherapy in lung cancer. The experts’ comments reflect the fact that targeted therapies have changed the standard of care for stage III and IV non–small-cell lung cancer (NSCLC) from nonspecific cytotoxic chemotherapies to precision treatments in select patients based on the identification of predictive molecular biomarkers targetable by specific agents.2 However, there are still challenges to overcome in successfully implementing biomarker-driven targeted therapy in clinical practice.3 Clinically useful molecular biomarkers can be divided into gene fusions or gene amplifications (tested at the cellular level by immunohistochemistry or at the cytogenetic level by fluorescence in situ hybridization), and gene mutations (eg, EGFR mutations).2,4 Moreover, screenings that include RAS, BRAF, MET, ERBB2, ALK, ROS, and PD-L1 have been shown to be useful in treating patients with lung cancer, such that targeted NGS is now replacing single-gene testing methods (Table 1).2,4,5 These developments require the entire cancer care team to work together to ensure that appropriate tissue samples are collected and requests for biomarker testing are clear and grounded in evidence-based practice. Dr Herbst and Dr Rimm discussed the various ways that oncologists and pathologists can work together to optimize this process.
Best Practices in Communication Among the Oncologist-Pathologist Team
Communication among the cancer care team is critical for successfully implementing molecular biomarker testing in patient care, particularly in lung cancer. Dr Herbst noted, “Communication among the medical oncologist, pathologist, surgeon, pulmonologist, radiologist, and the team that is obtaining the biopsy is critical. It is crucial to know which markers are important and to have that information up front. It is also an ever changing field. We are very fortunate at Yale Cancer Center to have processes that facilitate communication between Dr Rimm and myself. We have tumor boards on a routine basis where we present cases, including a pathology review, a radiology review, and a clinical discussion. We also have a lung cancer research working group called DART (Disease Aligned Research Team). We meet on a weekly basis, and discuss the latest biomarkers in lung cancer that are clinically significant. We realize that there is a panel of markers that is needed for basic clinical care. I would like our center to be one where the patient gets the most up-to-date care that includes EGFR, ALK, ROS, RET, MET, BRAF, and HER2. These are targets we have drugs for, but there are many things that are still in the investigational stage that are also important to know. We can do a whole exome, if that is necessary. If tumor mutational burden [TMB] is relevant, we can do that as well. Dr Rimm works closely with us, both in the clinical and research setting, as part of our lung cancer program.”
Dr Rimm added, “We are taking the things that we are discovering in the basic laboratories and moving them to the clinic. That is where we interact, informally and formally, in terms of presenting to the oncologists what is new and how we are going to improve patient care. It goes both ways: from the patient side, we work toward developing assays that are used on a regular basis and from the research side, we are working on developing assays that can be tested in the clinical setting.”
The importance of communication among oncologists, pathologists, and other members of the cancer care team is stressed at other institutions as well, as Dr David Spigel, MD, Chief Scientific Officer and Director of the Lung Cancer Research Program at the Sarah Cannon Research Institute, Nashville, TN, explained. “When I first started caring for patients, I worked with a pathologist, but we did not work together very closely. It was mainly a one-way interaction in which I received a report, and occasionally I would call if I needed further explanation. It is different now. It is routine to have regular interactions in a multidisciplinary fashion. Surgeons, radiation oncologists, medical oncologists, and pathologists work together to make decisions on patient care. We routinely engage our pathologists to help us bring in new discoveries. Meetings such as ASCO [American Society of Clinical Oncology], for example, afford an opportunity to hear about a marker that could be the next greatest marker for a particular cancer. Conversations with our pathologist about setting up that marker at our institution are important. Utilizing a new assay, if we think it is ready for prime time, if it is validated, is actually a part of cancer care now. That is a big shift. A decade ago I never used to have these conversations. Now it appears as though every few months we discuss bringing new assays into the house and broadening the way we do testing. How do we make it all happen? Most often, it is at our tumor board. When we are presenting a case, there is usually an opportunity to mention a new study that came out, data on a novel therapy, or a new biomarker for that case.”
Dr Herbst added, “Who drives the bus regarding biomarker testing? I would say both the oncologist and the pathologist. It works in both directions. It is more like one of those big fire trucks, where you have a driver in the front and a driver in the back, and we take turns who is at which control. It has to work in both directions. The number 1 thing about any program is that you must meet the full needs of the patient. That is a 2-way street, and it is not just 2 people, it is the entire team. You must have a team that is all working together—oncology, pathology, radiology, interventional radiology, pulmonary, and the people that are obtaining biopsies. If your interventional radiologists do not know that they need to get frozen tissue, they will not get it. If they do not know you need 4 or 5 cores and they are satisfied with 1, you will not get it. There has to be a culture that encompasses the whole team, where communication among team members is vital.”
The Process of Biomarker Testing: Practical Issues
Although targeted therapy is the standard of care for patients with molecular target(s), and immuno-oncology therapy can be a standard for some subsets of patients, practical challenges remain in successfully implementing biomarker-driven therapy in clinical practice. In NSCLC, one such challenge is the infrequency of certain targetable mutations such as ALK and ROS1, necessitating testing for a broad range of biomarkers.5 As Dr Rimm explained, “The International Association for the Study of Lung Cancer [IASLC] has recently published a standard for biomarker testing.6 We want to be sure we are doing everything according to the standards, which is EGFR mutations. We pretty much look at the sequence for the whole cytoplasmic domain to catch every possible mutation that is there. Then we also do ALK and ROS. Our panel is a 50-gene panel that we routinely do—which also includes other markers, such as MET, RB2, RET, and BRAF—on every patient. That is the guideline. We are meeting the guideline and exceeding it a bit by doing all of these other genes. Then we also do PD-L1. That is part of the guideline as well, to do PD-L1 on every patient that comes in. That is kind of the starting point, but the starting point is changing. In fact, even how we report out the starting point is going to change. For example, we recently heard in an ASCO 2018 plenary session about changing the PD-L1 cut point,7 so we are going to have to change our report.”
The experts also had strong opinions regarding more recently discovered biomarkers, such as TMB. Dr Rimm stated, “TMB is still experimental. There has been a lot of hype around it, so there are people in oncology who think TMB is ready to go. It is not. It has been shown in a number of research studies to be very promising. Perhaps the most exciting thing about TMB is it is complementary to the PD-L1 test. We can see that, once TMB is standardized and we can provide it as a test that means the same thing at Yale as it does at other centers, we will use it. When we see the standardization studies come out, as we did a few years ago for PD-L1 (and the PD-L1 test saw broad adoption after we understood the standardization), I think the same thing is going to happen with TMB. We will see the standardization of TMB testing and then we will see adoption of the test, depending on its value in directing therapy. Right now, the value of the test has been shown in only a couple of trials. It is not very sensitive and specific, but it is about the same as PD-L1. The unique thing is that it is different. Maybe we will do both tests to maximize the number of patients we can put on targeted drugs.”
Dr Herbst added, “I think TMB is becoming more interesting by the day—the fact that patients who are PD-L1 negative or zero can still have high TMB. It does look like PFS [progression-free survival] and response rates to combinations of drugs such as PD-1 and CTLA-4 inhibitors appear to be good. We are still waiting for some more maturation of response and survival data. If we saw that, I would have to give Dr Rimm a call and say, ‘We have to do TMB; we have to figure that out.’ There is a national effort underway to standardize TMB, but it is tricky. What do you consider a mutation? How many genes do you test? How does it correlate? Then there are so many other things that will be coming into play.”
Another major challenge in biomarker testing is acquisition of adequate and appropriate tumor tissue. The accuracy of molecular tests is affected by both the quality and the quantity of the tumor tissue obtained, placing great emphasis on the importance of collecting and processing the samples.3 Educating physicians who are performing biopsies about the importance of obtaining a sufficient amount of tumor tissue is critical in meeting this challenge. For example, molecular testing of lung adenocarcinoma is usually requested on small biopsy specimens obtained by either core needle biopsy (CNB) or fine-needle aspiration (FNA) biopsy. CNB yields a larger tissue specimen and provides a significantly higher number of samples sufficient for molecular testing than does FNA (in one study, 67% vs 46%, P =.007).8 Therefore, radiologists performing CT-guided lung biopsies should be encouraged to use CNB rather than FNA. This may be accomplished by using shared protocols or accessing existing guidelines.
The relative lack of physicians’ knowledge and consensus on molecular testing may be overcome by regular multidisciplinary meetings or molecular tumor boards.9 Developing general molecular testing policies and procedures at the institutional level, based on available guidelines, is also helpful because it may improve workflow efficiency and testing performance.10
Dr Rimm noted, “The amount of tissue is critical. In fact, if the biopsies are small, we can make nucleic acids from them. However, if we make nucleic acids, we cannot test for PD-L1 or other markers that say whether it is an adenocarcinoma or a squamous cell. You have to triage the amount of tissue you get. We have to do all of the standard-of-care tests first. We may get only 20 sections from a standard biopsy, and then it is gone.”
Liquid biopsy, or using blood or plasma samples, is an alternative to tissue biopsy; it can even replace tissue biopsy in cases where biopsy or cytology samples are not available.11,12 When asked whether liquid biopsies are a possible solution to limited tissue for biomarker testing, Dr Rimm responded, “Liquid biopsies are interesting because they allow you not only to get more tissue, because you just draw more blood, but to get it at multiple time points. Interesting studies are now going on to try to evaluate the value of liquid biopsy. In some targeted therapies, there is already clear use for liquid biopsies. Even in the 2018 guidelines from our societies, it is recommended to use liquid biopsy when you cannot acquire tissue.6 Will it give us the sensitivity and specificity we need? Those trials still need to be done.”
Dr Herbst added, “On our lung cancer master protocol, we are now obtaining liquid biopsies. I use a liquid biopsy in the clinic when I want something quickly. It can be done in real time. However, if it is negative, you still need to go to the tissue. If you do targeted liquid biopsies, results come back really quickly. Very often when someone has an EGFR-mutated disease that is recurrent, there are not easy tissue sites to biopsy. Liquid biopsies can be very useful. They are going to be the future, and the technology keeps getting better every year.”
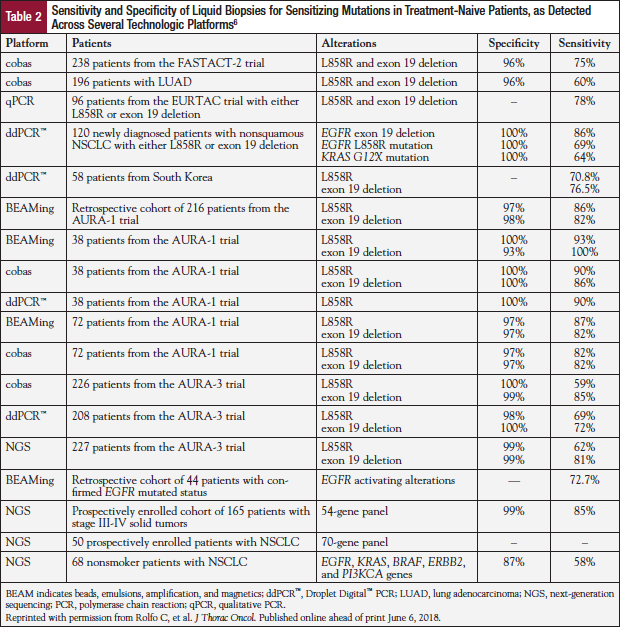
Although liquid biopsy has the potential to overcome some of the challenges encountered with pathologic assessment of tissue biopsies—because it is minimally invasive, can be repeated easily, and can potentially provide a more comprehensive molecular profile of tumors at multiple metastatic sites—this approach currently lacks the required sensitivity with high positive predictive value and low negative predictive value (Table 2).6 Moreover, there may be insufficient circulating free tumor DNA in plasma to identify any mutations.13,14
The time from tissue acquisition to reporting of results is also a significant issue that impacts the practical application of biomarker testing in clinical practice. As Dr Rimm pointed out, “Time is a critical factor. We would like to turn everything around in 24 hours, but we cannot. Even in an immunohistochemistry test, we usually have a 48- to 72-hour turnaround time. The problem with NGS is that the process itself takes 7 to 10 days. That means that we are talking 2 to 3 weeks before we really convey all of the necessary information to the oncologists. There are several groups trying to speed that up, and there are numerous technologies that may increase the speed of learning about a small panel of genes or even larger panels of genes. Right now, it is the time of the actual test itself—the time that it takes to actually do the physical steps that are required to get the information and then analyze the data—that is hard to do very quickly for that much sequenced data. Immunohistochemistry data and then protein-based data that look at the tumor micro-environment—those should all be turned around in 48 to 72 hours.”
Dr Herbst added, “Timing is crucial because patients are waiting for therapy. I think as long as we know things are moving forward, that is reassuring. I will often call the pathology laboratory and ask, ‘What is happening with the sample?’ It is not just the time to process; it is also acquiring the proper tissue samples and the time to get the tissue from the patient to the laboratory. That is a topic of conversation at practically every tumor board.”
Cost and Value in Biomarker Testing
Flowers and Veenstra have provided a framework for assessing the cost-effectiveness of a pharmacogenomic strategy in cancer by relating certain features of a testing strategy (eg, prevalence, penetrance, sensitivity, specificity, cost, prevalence, and treatment outcomes) to the cost-effectiveness of each measure.15,16 For example, testing for a certain gene is more cost-effective when the variant allele of the gene is relatively common in certain malignancies. Moreover, a molecular test is more cost-effective when it has high specificity and sensitivity for a targetable mutation. Importantly, pharmacogenomics testing that positively affects clinical outcomes by increasing efficacy and improving quality of life while reducing the occurrence of adverse events is considered cost-effective using this analysis.15-17
This framework can assist pharmacists, physicians, and policymakers in evaluating the implications of specific pharmacogenomic strategies. Nevertheless, each oncology practice must determine the current state of payer reimbursement for biomarker tests (including NGS). Which tests are authorized and covered more easily than others? Does ease of obtaining authorization and coverage vary by tumor type? Which payers (Medicare vs commercial vs managed care) are relatively more “generous” and straightforward than others? What steps are involved in preauthorizing biomarker testing? Each practice also needs to develop standard operating procedures for preauthorization and payer interface. It is vital for each practice to be aware of the steps their affiliated cancer center has taken to address biomarker-specific, payer-related challenges. Have oncology and pathology collaborated to develop a set of reflex tests and specific testing protocols? Does oncology or pathology order biomarker tests? Does the pathology administration educate pathologists and oncologists about payer access and reimbursement issues to streamline the process?
Dr Rimm provided his perspective: “Money and reimbursement of tests have been a big problem because the payers do not want to reimburse until the test has been proven to be necessary. Then they want to reimburse for what they think it should cost. That may or may not be the real world.”
Dr Herbst added, “Certainly, what is needed is for the current standard of care to be reimbursed, and we have to continue to fight for that. If you can pull along with it other genes that are evolving, I think that is very important.”
Conclusions: Applying Best Practices in Biomarker Testing
The experts summed up their perspectives on the benefits and challenges of biomarker testing in oncology. Dr Herbst concluded, “Biomarker testing has been quite successful, but there remain many challenges. As drugs are moving forward, we are going to have to use biomarkers to personalize therapy in appropriate ways. One of the biggest challenges, of course, is availability of biopsies and making sure that if we are going to be performing biopsies on all patients, we have the technical capacities to do that. In lung cancer, we do CT-guided biopsies, we can do bronchoscopy with endobronchial ultrasound [EBUS] bronchoscopy. It is a lot of time and waiting, and that adds to the time you can treat. Then, of course, there is having enough tissue and prioritizing the tissue. That is going to require multiplexing the tests. We need an X gene platform that gets as many tests as possible, but it is not all going to be a DNA sequencing test. There are other things that need to be done as we have discussed—proteins and immunochemistry and other things that are going to require adequacy of tissue. Liquid biopsies can certainly help in that regard. That is going to be important. These things have to be done in a reasonable time frame as things come forward that take a little bit longer, such as TMB. We have to figure out how to do that. It may mean we have to get these tests before we are ready to treat the patient. Finally, cost and approval—we have to design methods and studies so that these tests can be associated with the outcomes, and that is not as easy as it appears. A lot of challenges, but what an exciting time.”
Dr Rimm made the following closing statement. “I would argue that we have not yet seen the biggest problem, but it is coming down the pike. We heard at ASCO how in melanoma, the patient is pretty much cured by the surgery, but now they are giving the drug just in case they missed some cells. That adjuvant setting is where you really need a companion diagnostic test. If you look at the data from melanoma, we found that only 1 in 5 patients benefits from the drug in the adjuvant setting. That is, if you look at the patients who only received placebo, 50% of them do just fine. In the patients who received the drug, 30% do not benefit from the drug anyway. That is the real question. How do we identify the 80% who are not going to benefit or are doing fine from the surgery as opposed to the 20% who are actually benefiting from the drug? That is where the diagnostic tests and companion diagnostics will be even more important. I am working on that in preparation, because lung cancer is going to be next. I am sure that we are going to be giving immunotherapy in the adjuvant setting in low-stage lung cancer to prevent recurrences. On the one hand, that is a great thing to do; on the other hand, you do not want to give a drug to 10 patients to benefit 1, especially when 1 or 2 will lose their thyroid. You do not want to give a drug unless the patient really needs it. That is one of the biggest future challenges—diagnostic tests with high sensitivity but also high specificity, so that we can give the drug in the adjuvant setting.”
When asked about best practices that can be gleaned from the Yale Cancer Center experience, Dr Rimm responded, “One of our best practices that we think is critical is trying to have rapid turnaround of the key information for the oncologists. We want our oncology team, when they see that patient, to already know the mutation status and to already know what the PD-L1 status is. Those are the 2 biggies: the mutation status and the PD-L1. In terms of the timing, we want our PD-L1 to be turned around in 72 hours. We would like our mutation status to be turned around in 10 days.”
Dr Herbst added, “What is more important is interpretation, knowing what to do with those results. Why send a test if you are not going to act on it? What we try to do is educate at tumor boards, in meetings at our center, for those who are in the community. This can be done through meetings they attend or perhaps through educational forums. When asked what the biomarkers mean or how you use them to inform therapy, I would expect that whoever does your marker, that group has ways of communicating with you about what the results mean. Let’s say you do a TMB—what does it mean? You may want to call someone at the center and ask them, ‘How do you interpret this?’ The more communication you have, the better. Education and communication are key—everyone on the team must always remember that there is a patient on the other end waiting for a result that is going to affect how his or her disease is treated.”
References
- Nalejska E, Mączyńska E, Lewandowska MA. Prognostic and predictive biomarkers: tools in personalized oncology. Mol Diagn Ther. 2014;18:273-284.
- Garinet S, Laurent-Puig P, Blons H, Oudart JB. Current and future molecular testing in NSCLC, what can we expect from new sequencing technologies? J Clin Med. 2018;7:E144.
- Lee DH. Practical issues of biomarker-assisted targeted therapy in precision medicine and immuno-oncology era. ESMO Open. 2018;3(suppl 1):e000370.
- Luk PP, Selinger CI, Mahar A, Cooper WA. Biomarkers for ALK and ROS1 in lung cancer: immunohistochemistry and fluorescent in situ hybridization. Arch Pathol Lab Med. 2018;142:922-928.
- Ahmadzada T, Kao S, Reid G, et al. An update on predictive biomarkers for treatment selection in non-small cell lung cancer. J Clin Med. 2018;7:E153.
- Rolfo C, Mack PC, Scagliotti GV, et al. Liquid biopsy for advanced non-small cell lung cancer (NSCLC): a statement paper from the IASLC [published online ahead of print June 6, 2018]. J Thorac Oncol. doi:10.1016/j.jtho.2018.05.030.
- Lopes G, Wu Y-L, Kudaba I, et al. Pembrolizumab (pembro) versus platinum-based chemotherapy (chemo) as first-line therapy for advanced/metastatic NSCLC with a PD-L1 tumor proportion score (TPS) ≥ 1%: open-label, phase 3 KEYNOTE-042 study. J Clin Oncol. 2018;36(suppl). Abstract LBA4.
- Schneider F, Smith MA, Lane MC, et al. Adequacy of core needle biopsy specimens and fine-needle aspirates for molecular testing of lung adenocarcinomas. Am J Clin Pathol. 2015;143:193-200.
- van der Velden DL, van Herpen CM, van Laarhoven HW, et al. Molecular tumor boards: current practice and future needs. Ann Oncol. 2017;28:3070-3075.
- Cree IA, Deans Z, Ligtenberg MJ, et al. Guidance for laboratories performing molecular pathology for cancer patients. J Clin Pathol. 2014;67:923-931.
- Schwarzenbach H, Hoon DS, Pantel K. Cell-free nucleic acids as biomarkers in cancer patients. Nat Rev Cancer. 2011;11:426-437.
- Crowley E, Di Nicolantonio F, Loupakis F, Bardelli A. Liquid biopsy: monitoring cancer-genetics in the blood. Nat Rev Clin Oncol. 2013;10:472-484.
- Ettinger DS, Wood DE, Aisner DL, et al. Non–small cell lung cancer, version 5.2017, NCCN Clinical Practice Guidelines in Oncology. J Natl Compr Canc Netw. 2017;15:504-535.
- Pirker R. Molecular biomarkers in advanced non–small-cell lung cancer: a rapidly changing field. J Oncol Pract. 2017;13:231-232.
- Flowers CR, Veenstra D. The role of cost-effectiveness analysis in the era of pharmacogenomics. Pharmacoeconomics. 2004;22:481-493.
- Goldstein DA, Shaib WL, Flowers CR. Costs and effectiveness of genomic testing in the management of colorectal cancer. Oncology (Williston Park). 2015;29:175-183.
- Flowers CR, Veenstra D. Will pharmacogenomics in oncology be cost-effective? Oncol Econ. 2000;1:26-33.